Cancer Immunotherapy
The fields of immunology and oncology have been linked since the late 19th century, when the surgeon William Coley reported that an injection of killed bacteria into sites of sarcoma could lead to tumor shrinkage.
But over the past several years, immunotherapy—therapies that enlist and strengthen the power of a patient’s immune system to attack tumors—has emerged as what many in the cancer community now call the “fifth pillar” of cancer treatment.
In 2017, two CAR T-cell therapies were approved by the Food and Drug Administration (FDA), one for the treatment of children with acute lymphoblastic leukemia (ALL) and the other for adults with advanced lymphomas. Nevertheless, researchers caution that, in many respects, it’s still early days for CAR T cells and other forms of ACT, including questions about whether they will ever be effective against solid tumors like breast and colorectal cancer.
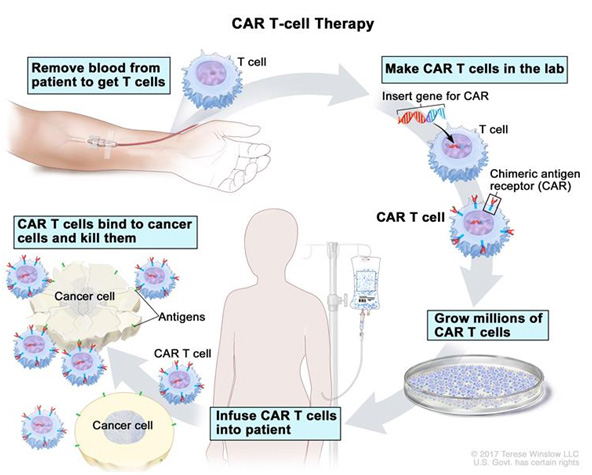 Chimeric antigen receptor (CAR) T cells are genetically modified T cells, where a patient’s own (autologous) T cells are manipulated ex vivo to express the antigen-binding domain from a B cell receptor that is fused to the intracellular domain of a CD3 TCR (CD3-zeta).
Chimeric antigen receptor (CAR) T cells are genetically modified T cells, where a patient’s own (autologous) T cells are manipulated ex vivo to express the antigen-binding domain from a B cell receptor that is fused to the intracellular domain of a CD3 TCR (CD3-zeta).
CAR T cells have been studied most extensively in hematologic malignancies. Clinical trials targeting CD19, the pan-B cell antigen, have shown remarkable success in B cell acute lymphoblastic leukemia (B-ALL) and pre-B-cell ALL . Trials in patients with chronic lymphocytic leukemia (CLL) have also shown promising results.
Numerous trials in hematologic malignancies are ongoing, with early development in some solid tumors targeting shared antigens such as CEA, mesothelin, and HER2 are also underway.
Our collaboration with World leaders in CAR T Cell Therapy Cancer Treatment reflects our shared commitment to bringing the first marketed CAR-T cell therapy treatment option currently approved in the United States, European Union and Canada for Indians to benefit from within Asia.
Cancer Immunotherapy and stare of the art revolutionary therapies:
World over clinicians and experts are promising moonshots to treat or find cures for several cancer types prevalent across countries and some others that are rampant and have no selection criteria across peoples of the world – they attack all economy, race and worlds.
Revolutionary technologies such as Immunotherapy in cancers and its advancements such as CAR T cell therapy has been lately approved by US FDA and is a standard line of treatment for several cancers now with a promise to treat some cancers, almost miraculously and rapidly.
AceProbe takes pride in helping patients to select from destinations and experts in Asia to help gain this advantage with Doctors and Specialist trained in the leading centers of the Unites States of America helping candidates realize the same treatment at one third of the American costs, on demand.
Currently the CAR T Cell therapy is approved globally for:
- Refractory Acute Lymphoblastic Leukemia
- Relapsed or Refractory Diffuse Large B Cell Lymphoma
- Relapsed or Refractory Follicular Lymphoma
- Effective and responsive in
- Relapsed leukemias and lymphomas after HCT
- Relapsed or refractory DLBCL (diffuse large B cell lymphoma)
- Undergoing investigation for other hematologic malignancies such as chronic lymphocytic leukemia (CLL)
- Resulted in impressive responses in patients with relapsed refractory myeloma

